Safe Hydrogen uses a slurry of Magnesium/Magnesium Hydride particles with light mineral oil as a rechargeable medium. When the slurry is discharged, the particles are magnesium and, when the slurry is charged, the particles are magnesium hydride.
The chemical equation is: Mg + H2 <--> MgH2
The two way arrow indicates that the reaction is reversible which makes the slurry rechargeable. The atomic weight of the magnesium atom is 24 and the atomic weight of the hydrogen atom is 1. The molecular weight of MgH2 is 26. Hydrogen is 2 of the 26 or 7.7% by weight. We use a 50% mineral oil and 50% MgH2 mixture for the slurry. The magnesium hydride slurry has 3.85% hydrogen by weight.
It takes 12 kg of magnesium to store 1 kg of hydrogen. The 13 kg of MgH2 needs 13 kg of mineral oil to make a 50% slurry. Using today's (May 2021) prices of $1.96 per kg of magnesium and $1 per kg of mineral oil, the raw material cost to store 1 kg of hydrogen is $36.52. One kilogram of hydrogen burned in a gas turbine will produce 14 kWh of electricity. Thus, the raw material cost to store 1 kWh of electricity is $2.61 per kWh. Assuming that the cost of processing the magnesium and oil into a slurry increases the cost to store 1 kWh by a factor of two, the capital cost to store one kWh of electricity is approximately $5.22.
A capital cost of $5.22 to store 1 kWh of electricity compares extremely favorably with the capital cost of batteries, which ranges from $200 to $500 per kWh stored.
A similar calculation can be made to see what the cost is to move a kilogram of hydrogen from point A to Point B. The cost of a trailer to transport 34,000 kg of slurry is about $100,000. The cost of the truck to pull that trailer is about $90,000. Assuming the cost of the driver is about $62,500 with benefits and that the truck can be filled and emptied 2 times a day, the cost of delivery would be about $0.20/kg of hydrogen delivered not counting the cost of the slurry that is circulating.
Next we estimate the total cost of the system, including the slurry. Using the same raw material and processing costs of $76.4 per kg and a 200 km round trip, the cost is highly dependent upon how many round trip cycles the slurry can undergo before it has to be replaced. Safe Hydrogen has cycled the slurry 50 times without any degradation in its hydrogen carrying capacity. It is likely that the particles will retain their carrying capacity for thousands of cycles. At 50 cycles, the cost of delivery is $1.73/kg of H2; at 100 cycles, the cost is $0.96/kg of H2; and at 1000 cycles, the cost is $0.28 per kg of hydrogen delivered. These figures are just the cost to move the hydrogen. One must add the production cost of the hydrogen. Production costs for hydrogen produced by the steam reformation of hydrogen or by solar assisted electrolysis is in the range of $1.00 to $1.50 per kg. One kg of hydrogen has approximately the same energy content as a US gallon of gasoline. Therefore, it is apparent that the delivered cost of hydrogen can be competitive with the retail price range of gasoline in the United States, which is currently (Nov 2015) in the range of $1.75-2.50 per gallon.
|
Charging
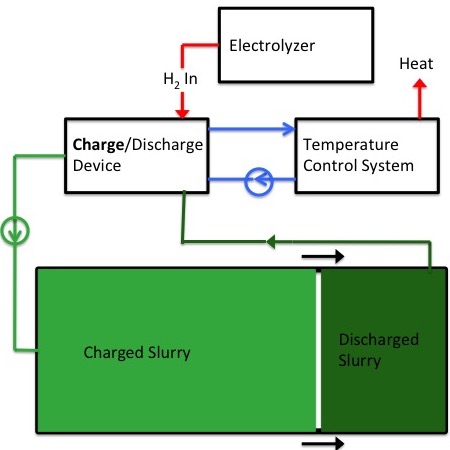
|
|
Discharging
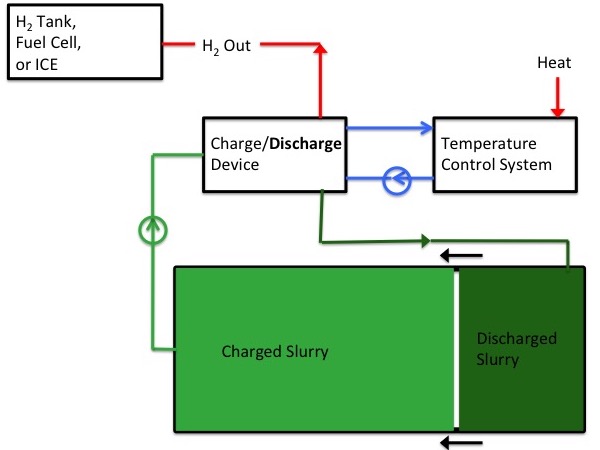
|
The hydrogen storing ingredient of the Safe Hydrogen chemical hydride slurry is a metal which had been converted to a metal hydride in the hydrogen "storing process". The metal hydride releases it's hydrogen, when required, in a managed reaction with water. The reaction, besides producing hydrogen, generates some heat and converts the slurry metal from a metal hydride to a metal hydroxide. This "metal hydroxide" slurry is then totally recycled including the conversion of the metal hydroxide back to a metal hydride. The recycling and conversion is achieved in a very large scale specialized process before the slurry can be used again.
The chemical equation for this reaction is: MgH2 + H2O --> MgO + 2H2
The arrow indicates that reaction is one way or irreversible. For the same amount of magnesium hydride, this reaction gives twice the hydrogen as does the rechargeable slurry. The two H2 molecules represent 15% of the weight of the magnesium hydride. Because the reaction temperature is a moderate 100oC, we use a slurry with 70% solids. The hydrogen released is 11% of the slurry weight.
The process to reduce the magnesium oxide (MgO) back to magnesium and to hydride the magnesium is expensive. In very large volume, the cost of the hydrogen released would be about $5.00 per kg. In small volume, the hydrogen would cost about $30 per kg.
With the larger weight % released, this technology is best for autonomous vehicles, such as unmanned, underwater, devices. Out technology can provide up to weeks of electrical power when coupled with a fuel cell.
The hydrogen storage and release reactions take place in specialized proprietary devices developed and designed by Safe Hydrogen and scaled to the appropriate process. The slurries and devices are patent protected.
|
Chemical Hydride System
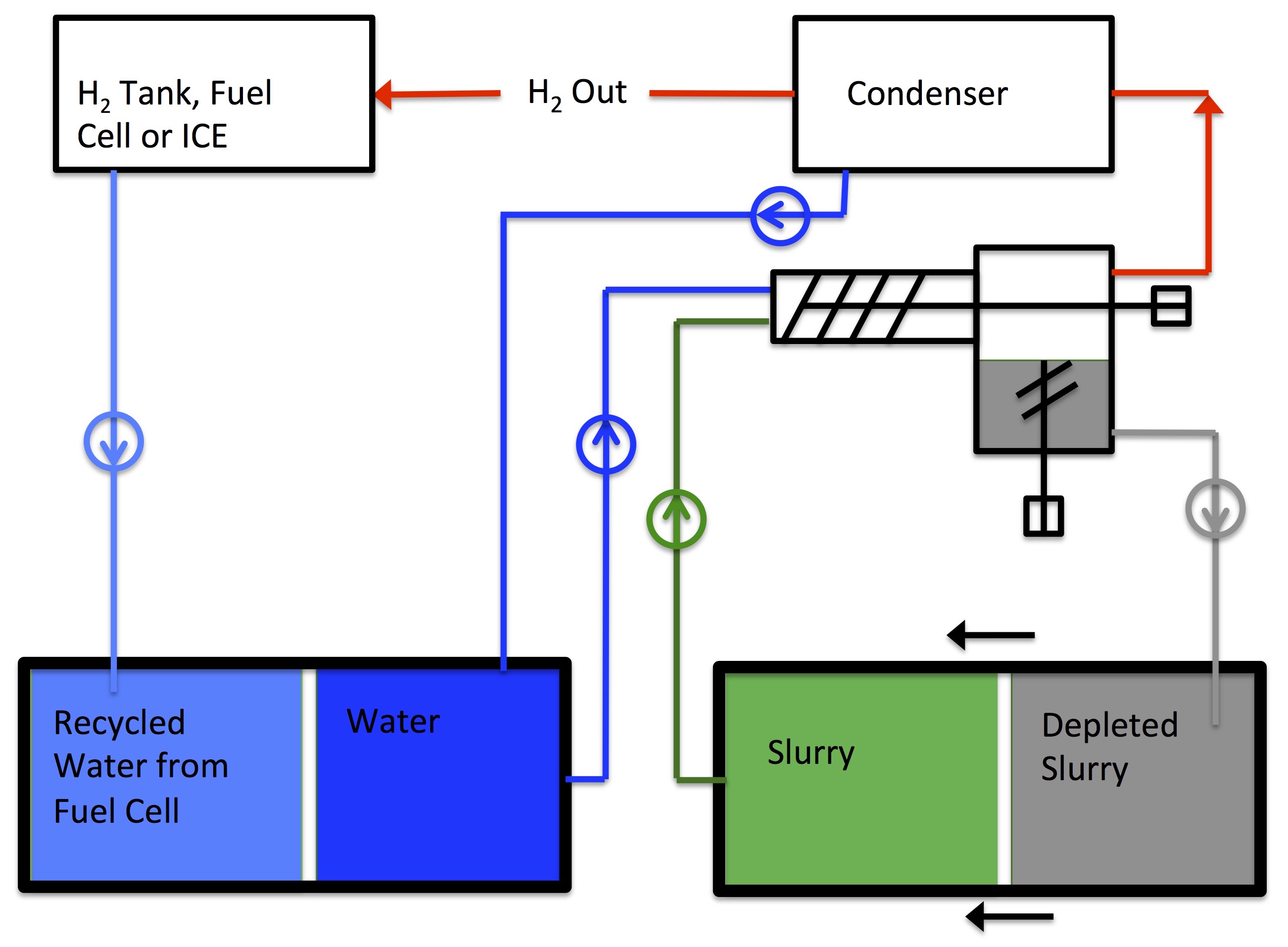
|
|

Efficient and safe storage and delivery of hydrogen is the major technical challenge of utilizing hydrogen as an alternative energy carrier.
Compressed and liquid hydrogen tanks often don't store sufficient volumes and pose safety risks. But Safe Hydrogen's metal hydride based chemical hydride and rechargeable hydride technologies offer storage efficiency and storage safety while providing the cost saving advantage of being able to use the existing fossil fuel infrastructure to deliver and store a pumpable and non-explosive hydride slurry as future "hydrogen fuels."
Cryogenically cooling hydrogen into a liquid state is a well established technology and considered the comparative benchmark for storing hydrogen. But the technology requires substantial energy to liquefy the hydrogen, includes continual "boil off" of hydrogen during storage and requires very costly storage tanks and handling.
Slurries, on the other hand, are stored at normal temperature and
pressure, can use existing low-cost "fossil fuel" tanks and pipe
lines and because they are not explosive and are flame resistant,
they do not require any specialized and costly handling procedures.
Below, we show the results of holding a blow torch on a blob of slurry.

|





